D1.jpg) Gen info Gen info
- Alangium is a small genus of flowering plants included either in a broad view of the dogwood family Cornaceae or as a sole member of its own family Alangiaceae. The genus has about 40 species, some with unclear boundaries.
It consists of small trees, shrubs and lianas. Most of the species are native to tropical and subtropical regions of east and southeast Asia.
(3)
-
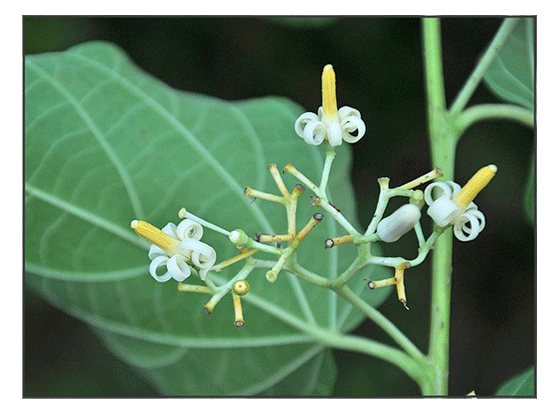
Alangium chinense is a species of flowering plant in the Cornaceae family.
- Etymology: The genus name Alangium is a Latinization, derived from the Malayalam name alangi. It was named in 1783 by Jean-Baptiste Lamarck in his Encyclopedie Methodique. (3)
Botany
Alangium chinense is a shrubs or small tree, 3-5 m tall. Branchlets pubescent when young, glabrescent. Petiole reddish, 4-6 cm; leaf blade ovate or orbicular to cordate, 8-20 × 5-12 cm, abaxially tufted pubescent at axils of veins, adaxially glabrous, strongly 3-5-veined at base, base usually oblique, occasionally rounded or subrounded, or triangular, margin entire or with few shallow lobes, apex acuminate. Inflorescences axillary cymes, 3-15-flowered. Flowers ca. 2 cm. Calyx lobes 4-7, shortly dentate. Petals valvate, 6(-8), lanceolate, 1-1.5 cm. Stamens 6-8, ca. as many as petals, glabrous. Drupe ovoid, 5-7 mm, seed 1. (Flora of China)
Distribution
- Native to the Philippines.
- Also native to Angola, Assam, Bangladesh, Burundi, Cabinda, Cambodia, Cameroon, China, Ethiopia, Gulf of Guinea Is., Himalaya, India, Jawa, Kenya, Laos, Lesser Sunda Is., Malawi, Mozambique, Nepal, Rwanda, Sudan, Taiwan, Tanzania, Thailand, Tibet, Uganda, Vietnam. Zambia, Zaire, Zimbabwe.
(2)
- Grows primarily in seasonally dry tropical biome.
(2)
 Constituents Constituents
- Study of roots isolated a new phenolic glycoside, chinenside A (1), a new megastigmane glycoside, chinenionside A (2), along with 12 known compounds (3-14). (see study below) (8)
- Study of ethanol extract of roots isolated four new sesquiterpenes (1-4), four new alkaloids (5a, 6a, 6b, and 7), and nine known compounds (5b, 8-15). (see study below) (9)
- Study of ethanolic extract of roots isolated two new norditerpenoids (1 and 2), four new sesquiterpenoids (3-6), and 22 known compounds (7-28).
(see study below) (11)
- of ethyl acetate layer of fibrous root of A. chinense isolated a new sesquiterpene, 1-carbonyl-2,8-dihydroxy-11-oxabicyclo [4,4,1] germacra- 2(3),4(5),6(7),8(9)-tetraene (1) and four known compounds (3E, 23E)-3-caffeoyl-23-coumaroylhederagenin (2), (3E, 23E)-dicoumaroylhederagenin (3), morettinone (4), 24-ehylcholesta-3,6-dione (5). (12)
- Study of dried leaves of A. chinense isolated five novel phenolic glycosides, 6‘-O-galloylsalicin (1); 4‘,6‘-di-O-galloylsalicin (2); 4‘,6‘-O-(S)-hexahydroxydiphenoylsalicin (3); 4‘,6‘-O-(R)-hexahydroxy-diphenoylsalicin (4); and pyrocatechol 1-O-β-d-xylopyranosyl(1→6)-β-d-glucopyranoside (5). (17)
- Study of roots isolated a new isoflavonoid, alangchnin A, together with two known compounds (2 and 3).
(19)
Properties
- Roots and stems considered blood tonic, carminative, contraceptive, anti-rheumatic, vulnerary.
- Studies have suggested anti-inflammatory, anti-rheumatoid arthritis, antipyretic, analgesic, anticancer, neuroprotective, antioxidant, neuritis inhibitory, antiviral, antitumor properties.
 Toxicity Toxicity
- Toxic constituent: Anabasine.
- Toxic dose: Reported lethal dose: 80 g fresh fibrous roots (prepared into herbal decoction with wine).
- Mechanism: Anabasine is an agonist at nicotinic cholinergic receptors. Toxicity due mainly to stimulation of sympathetic ganglia; with larger doses or prolonged exposure, paradoxical inhibition occurs, resulting in ganglionic and neuromuscular blockade.
- Symptoms and manifestations: Early: dizziness, nausea, tachycardia, hypertension, muscle twitching. Late: bradycardia, hypotension, weakness, paralysis, respiratory failure.
- Case Report: 50-yr old man in China developed limb weakness and respiratory failure after drinking a herbal decoction prepared from a large dose (80g) of fresh fibrous roots with wine for knee pain.
- Treatment: Supportive. (Atlas of Poisonous Plants in Hong Kong) (21)
Parts used
Roots, flower, leaves.
Uses
Folkloric
- No reported folkloric medicinal use in the Philippines.
- In Chinese herbalism, considered one of the 50 fundamental herbs.
- In traditional Chinese medicine, used for snake bites, contraception, hemostasis, rheumatism, and wounds. Roots, flowers and leaves used for treatment of rheumatic arthritis, acroanesthesia, and fractures. Used for lumbago due to overstrain, numbness of the extremities, traumatic injuries, bleeding wounds, and snake bites.
- Used for pain and as muscle relaxant.
- Plant soaked in alcohol used for treating rheumatism, better than fresh plant alone.
- Decoction of leaf shoots used as tonic. Paste of roots applied to area around dislocated bones to help in setting.
- In African traditional medicine, root powder used to treat headache; root and leaf decoctions used as purgative and to treat stomach pain; decoction of leafy twigs drunk to treat mental illness. In DR Congo, bark macerations used to treat skin diseases and elephantiasis. (22)
Others
- Illuminant: Seed oil used to light lamps.
Studies
• Anti-Inflammatory / Antipyretic / Analgesic / Leaves: Study evaluated evaluated methanol and ethyl acetate extracts of dried powdered leaves of Alangium chinense for biologic activity in adult albino mice for acute toxicity studies and analgesic activity and Wistar rats for anti-inflammatory and anti-pyretic activity. Acute toxicity study showed not significant behavioral change or mortality at dose of 2000 mg/kbw after 14 days of administration, suggesting an LD50 >2000 mg/kbw. In anti-inflammatory evaluation, extracts showed reduction in carrageenan-induced paw edema in a dose dependent manner, with methanol extract showing 90.83% inhibition at 500 mg/kg, compared to 5 mg/kg indomethacin at 94.32% inhibition. Tail immersion testing showed central analgesic effect, with the higher dose showing similar effect to morphine. (4)
• Inhibition of COX-2 and Cancer Cells / Roots: Study isolated one new compound (14) along with 24 known compounds (1-13, 15-25) from fibrous roots of A. chinense. Compounds were evaluated by MTT assay for inhibition effects on COX-2 enzymes and cancer cells. Compounds 1-4, 13-14, and 16-18 showed good inhibition against COX-2 enzyme, with compounds 1, 13, 14, and 17 showing stronger inhibition than positive control, celecoxib. Molecular docking showed compounds 13, 17, and 18 belong to ellagic acids with good COX-2 inhibition. Compounds 1, 5, 13, and 21 showed cytotoxicity against HepG2 cells, compounds 2 and 21 against Caco-2 cells, and compound 20 against HeLa cells. (5)
• Neuroprotective against A. chinense-Induced Neurotoxicity / Jin-Gu-Lian: Alangium chinense and its representative components can cause ineluctable neurotoxicity. Study evaluated the detoxification potential of the compatible herbs in Jin-Gu-Lian formula (CH) on A. chinense-induced neurotoxicity. AC treatment significantly reduced levels of monoamine and acetylcholine neurotransmitters in rat brains, including acetylcholine, dopamine, DOPAC, HVA, norepinephrine and serotonin. Compatible herbs attenuated AC-induced neurotoxicity as evidenced by increased locomotor activity, enhanced grip strength, decreased frequency of AC-induced morphological damage in neurons, and reduction of neuron-specific enolase (NSE) and neurofilament light chain (NEFL) levels. The combination of AC and CH ameliorated AC-induced oxidative damage by modulating activities of SOD, GSH-Px, and total antioxidant capacity. Compatible herbs in Jin-Gu-Lian formula alleviated AC-induced neurotoxicity by amelioration of oxidative damage, preventing neurotransmitters abnormality, and modulating pharmacokinetics. (6)
• Amelioration of Rheumatoid Arthritis / Salicin: Rheumatoid arthritis is a chronic inflammatory disorder linked to oxidative stress of rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs). MTT, ELISA, and Wester Blot methods evaluated the effects and mechanism of salicin on inflammation and oxidative stress of RA-FLSs. Salicin significantly reduced cell viability, cytokines, and matrix metalloproteinases-1/-3 expression in IL-ß-induced RA-FLSs and inhibited RS production in IL-1ß-induced RA-FLSs. It reduced collagen-induced arthritic and suppressed oxidative damage indices. Salicin ameliorated rheumatoid arthritis, which as be associated with oxidative stress and Nrf2-HO-1-ROS pathways in RA-FLSs. (7)
• Anti-Inflammatory / Phenolic Glycosides / Roots: Study of roots isolated a new phenolic glycoside, chinenside A (1), a new megastigmane glycoside, chinenionside A (2), along with 12 known compounds (3-14). The compounds were evaluated for anti-inflammatory activity in vitro against lipopolysaccharide-induced mouse macrophage RAW264.7 cells. Compounds 1, 9, and 10 potentially inhibited production of nitric oxide (NO), prostaglandin (PEG2), TNF-α, IL-1ß, and IL-6. Compound 1 showed stronger anti-inflammatory activity than Triptolide (TOL, 20 nm). (8)
• Antiviral / Antioxidant / Roots: Study of ethanol extract of roots isolated four new sesquiterpenes (1-4), four new alkaloids (5a, 6a, 6b, and 7), and nine known compounds (5b, 8-15). Compounds 3, 4, 8-13, and 15 exhibited antiviral activity against Coxsackie virus B3 with IC50s of 1.4-15.4 µM. Compounds 2-4, 7 and 9-13 showed antioxidant activities against Fe2+-cysteine-induced rat liver microsomal lipid peroxidation with IC50s of 3.8-45.7 µM. Compound 5b showed neuritis inhibitory activity against microglial inflammation factor with IC50 of 6.7 µM. None exhibited detectable toxicity against five tumor cell lines tested by MTT assay. (9)
• Antitumor / Pyridine Alkaloids: Study of Alangium chinense isolated three novel additive pyridine alkaloids, alangiumines A-C (1-3). Compound 1 exhibited selective antitumor in vitro activity against glioma stem cells (GSCs). Study suggests a potential new lead for the selective killing of human glioma stem cells. (10)
• Antiviral / Neuritis Inhibitory / Roots: of ethanolic extract of roots isolated two new norditerpenoids (1 and 2), four new sesquiterpenoids (3-6), and 22 known compounds (7-28). Compounds 1, 13, and 23 exhibited antiviral activity against coxsackie virus B3 with IC50s of 38-67 µM. Compounds 8 and 9 showed neuritis inhibitory activity against microglial inflammation factor with IC50s of 6.4 and 10.2 µM, respectively. (11)
• Anti-Inflammatory / COX-2 Inhibition / Terpenoids / Roots: Study of ethyl acetate layer of fibrous root of A. chinense isolated a new sesquiterpene, 1-carbonyl-2,8-dihydroxy-11-oxabicyclo [4,4,1] germacra- 2(3),4(5),6(7),8(9)-tetraene (1) and four known compounds (3E, 23E)-3-caffeoyl-23-coumaroylhederagenin (2), (3E, 23E)-dicoumaroylhederagenin (3), morettinone (4), 24-ehylcholesta-3,6-dione (5). Compound 1 showed good inhibitory effect against COX-2 with IC50 of 20.43 µM. Compounds 2-5 showed COX-2 inhibitory effect with IC50s of 49.19, 23.29, 47.78, and 44.44 µM, respectively. (12)
• Antiviral / Antioxidant / Phenolic Constituents / Roots: Study of ethanolic extract of roots isolated three new phenolics (1-3) and 28 known compounds (4-31). Compound 11 exhibited antiviral activity against Coxsackie virus B3 with IC50 of 16.89 µmol/L. Compounds 1, 10-17, 19-21, and 23 showed strong antioxidant activity against Fe2+-cysteine-induced rat liver microsomal lipid peroxidation, with IC50 values of 0.14-8.18 µmol/L. (13)
• Inhibition of Spontaneous Calcium Oscillations / Cadinane Sesquiterpenoids / Leaves: Study of leaves isolated nine new cadinane sesquiterpenoids, alanenses A-I (1-9) together with three previously reported analogues (10-12). Compounds 1 and 2 inhibit spontaneous calcium channel oscillations at low micromolar concentrations. (14)
• Effect on CYP450 Isoforms Activity: Study evaluated the effects of A. chinense on the metabolic capacity of cytochrome P450 (CYP) enzymes in rats, using a cocktail method to investigate activities of CYP2B1, CYP2D1, CYP1A2, CYP3A2, CYP2C11. Combined with PCR results, continuous 7-day intragastric administration of A. chinense inhibited the activities of CYP2D1 and CYP1A2 of rats. Enzyme inhibition by co-administered drugs and genetic variations of their expression can increase the risk of adverse reactions. (15)
• Anti AChE and HIV-1 Protease Activities: 2S (1a) and 2R-alanginenmine A (1b) from A. chinense exhibited anti-acetylcholinesterase (AChE) and HIV-1 protease activities in biological activity evaluation. Stronger Coulomb interactions and van der Waals interaction and hydrogen bond interactions of 1a, may be the main cause for its better anti-HIV-1 protease activity than 1b. (16)
• Inhibition of Neuronal Excitability: Study of leaves isolated two previously undescribed flavonols with phenylpropanoid or benzyl substitution, alangsine A (1) and alangsine B (2), along with four known compounds (3-6). The isolated compounds were evaluated for activity towards neuronal excitability. Compounds 1 and 2 inhibited neuronal excitability at low micromolar concentrations. Results suggested potential for treating pain and epilepsy. (18)
• Salicin Inhibition of AGE / Potential in Osteoarthritis: Osteoarthritis (OA) is a major age-related disease, which may be caused by accumulation of advanced glycation end-products (AGEs). Study investigated the effects of salicin,one of the main constituents of aspirin and a derivative of A. chinense, on AGE-induced degradation of the articular extracellular matrix in SW1353 human chondrocytes. Results showed a beneficial role of salicin in rescuing degradation of type II collagen and aggrecan, reducing oxidative stress, attenuating expression of proinflammatory cytokines, and inhibiting activation of the NF-kB proinflammatory signaling pathway in chondrocytes stimulated with AGEs. Study suggests potential for salicin as a safe and effective therapy against development and progression of OA. (20)
Availability
Wild-crafted.
|

![]()



D1.jpg)


