 Gen info Gen info
- Hyptis comprises about 250 species and is indigenous in the American tropics and subtropics. Some weedy species are found in the tropics of Africa, Asia, and Australia.
Botany
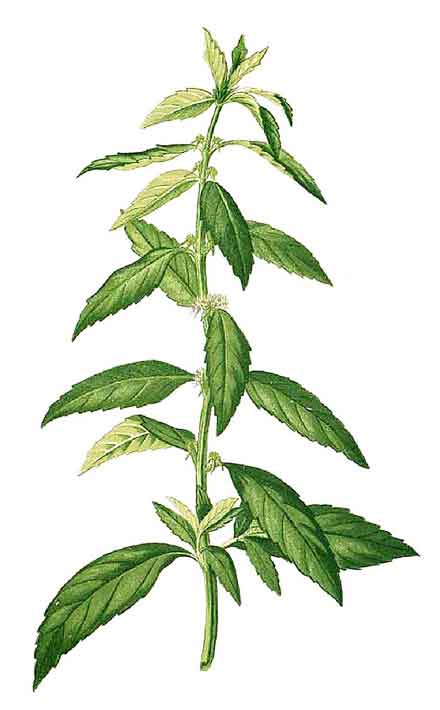
Hyptis brevipes is an erect annual plant up to 1 m high with the typical square stem of a labiate, often densely hairy but sometimes less so. Leaves are also normally coarsely hairy on both surfaces, opposite, narrowly ovate or lanceolate, 5–7 cm long, up to 2 cm wide, cuneate at the base, the margins irregularly serrate. Inflorescence is a dense raceme, almost globose, up to 14 mm diameter, on a peduncle about 1 cm long in the axils of most upper leaves. Corolla or petals are white, irregularly five-lobed, and about 5 mm long. Calyx, 4 mm long, also has five narrow, finely barbed lobes. Seeds are ovoid, up to 1 mm long, dark brown to black, obscurely striate, with a conspicuous scar. (2)
Herbs erect, annual. Stems 50-100 cm, angles appressed pilose. Petiole ca. 5 mm; leaf blade ovate-oblong to lanceolate, 5-7 × 1.5-2 cm, adaxially olive green, abaxially greenish, pilose, base narrowly cuneate, margin serrate, apex acuminate. Capitula axillary, ca. 1 cm in diam.; peduncle 0.5-1.6 cm, densely appressed pilose; bracts lanceolate to subulate, 4-6 mm, margin entire. Calyx subcampanulate, 2.5-3 × ca. 1.5 mm, minutely hispid; teeth as long as tube, apex subulate; fruiting calyx dilated. Corolla white, ca. 3.5 mm, puberulent, throat to 1 mm wide; upper lip ca. 0.5 mm, lobes circular, reflexed; middle lobe of lower lip larger, concave, circular, ca. 1 mm, constricted at base, recurved; lateral lobes triangular, reflexed. Stamens slightly exserted. Nutlets dark brown, ovoid, ca. 1 × less than 0.5 mm, adaxially ribbed, with 2 basal white scars. (Flora of China)
Distribution
- Introduced.
- Native range is Central Mexico to Tropical America.
(1)
-
In waste places up to 1,200 m altitude.
-
A weed in plantation crops, forest margins, often abundant in fallow rice fields. (4)
 Constituents Constituents
- Hydrodistillation and GC-MS study of H. brevipes for essential oils yielded 57 components in the leaf oil, with major components of germacrene D (13.54%), caryophyllene (12.31%), phthalamide doxime (9.47%) and caryophyllene oxide (8.57%). Inflorescence oil yielded 37 components with main components of caryophyllene oxide (45.09%), 1,5,5,8-tetramethyl-12-oxabicyclo[9.1.0]dodeca-3,7-diene (4.95%), caryophyllene (4.97%), and α-bourbonene (4.20%). (6)
- Study to isolate active metabolites from H. brevipes using Leishmania amazonensis strain for bioguided isolation yielded seven compounds: four brevipolides H, G, C, and J (1, 4, 6, 5); a catechol derivative (2), three flavonoids, chrysosplenol C (3), tomentin (7), and rutin (8). (see study below) (7)
- Bioassay guided fractionation identified two active compounds: 5-hydroxy-7,4'-dimethoxy-flavon-3-ol and 5- hydroxy-7-methoxy-2-(4`-methoxy-phenyl)-chromen-4-one. (see study below) (8)
- Study of entire plant isolated six new 5,6-dihydro-α-pyrone derivatives (1-6), namely, brevipolides A-F, together with seven known compounds, including 5,6-dihydro-α-pyrone derivative (7), three flavonoids, a steroid glycoside, and two triterpenoids. (see study below) (10)
Properties
- Studies have suggested antioxidant, antimicrobial, insecticidal, anticancer, larvicidal, antimycobacterial, anti-parasitic properties.
Parts used
Leaves, stems, oil.
 Uses Uses
Edibility
- Leaves sometimes eaten as vegetable.
Folkloric
- In Indonesia, leaves applied to wounds.
- In Malaysia, decoction of leaves and stems applied after childbirth. Poultice of leaves applied on abdomen of children to expel worms.
- In Central America, plant decoction used for treatment of headache and diarrhea.
- In Indonesia, leaves are applied to wounds.
- Essential oil used for treatment of asthma and malaria.
- In Bangladesh, leaf decoction used for diarrhea and fever. Paste of leaves or stems applied to forehead for relief of headache. Leaf juice taken for stomach disorders. Sugared leaf decoction used for skin diseases. (5)
Others
- Repellent: Essential oil used as mosquito repellent.
Studies
• Antimicrobial / Antioxidant / Essential Oil: Hydrodistillation and GC-MS study evaluated three Hyptis species i.e., Hyptis brevipes, H. suaveolens, H. rhomboidea for essential oil composition, toxicity, and biologic activities. Hyptis brevipes yielded 28 constituents. HB essential oil exhibited strongest antioxidant activity with SC50 2.019 µg/mL and strongest antimicrobial activity (3.125-6.25 µg/mL) on pathogens employed in the assay. H. brevipes showed significant toxicity with LC50 of 60.8 µg/mL. (3)
• Antiparasitic Metabolites: Study to isolate active metabolites from H. brevipes using Leishmania amazonensis strain for bioguided isolation yielded seven compounds: four brevipolides H, G, C, and J (1, 4, 6, 5); a catechol derivative (2), three flavonoids, chrysosplenol C (3), tomentin (7), and rutin (8). Compound 2 was most cytotoxic and active against all parasites tested. Brevipolide C (6) was most cytotoxic and active on Plasmodium flaciparum, while brevipolide H (1) was less cytotoxic and very active against Leishmania spp and G. lamblia, with weak activity against P. falciparum and T. cruzi. (7)
• Larvicidal / Insecticidal / Spodoptera littoralis: Hyptis brevipes exhibited strong insecticidal activity against 3rd instar larva of cotton leaf worm Spodoptera littoralis, inducing complete larval mortality due to arrest and/or disruption of metamorphosis. LC50 of the dichlormethane extract was 3.0% after three days of treatment. Bioassay guided fractionation identified two active compounds: 5-hydroxy-7,4'-dimethoxy-flavon-3-ol and 5- hydroxy-7-methoxy-2-(4`-methoxy-phenyl)-chromen-4-one. (8)
• Antimycobacterial: Study evaluated the antimycobacterial activity (M. a. massiliense) of six Brazilian medicinal plants and their fractions. H. brevipes showed a minimal bactericidal concentration of 1 mg/mL. After fractioning, ethanolic fraction of H. brevipes showed bactericial activity, and the ethyl acetate fraction showed antimycobacterial action. The best bactericidal function of all plant fractions was the ethanolic fraction, which contained rutin and rosmarinic acid shown to have microbiocidal activity. (9)
• Cytotoxicity / Cancer Cell Lines MCF-7 and HT-29 Cells: Study of entire plant isolated six new 5,6-dihydro-α-pyrone derivatives (1-6), namely, brevipolides A-F, together with seven known compounds, including 5,6-dihydro-α-pyrone derivative (7), three flavonoids, a steroid glycoside, and two triterpenoids.
Compounds 2, 6, and 7 exhibited ED50s of 6.1, 6.7, and 3.6 µM against MCF-7 cells, and compounds 1, 2, 6, and 8
(the known 5,6,3′-trihydroxy-3,7,4′-trimethoxyflavone) showed ED50s of 5.8, 6.1, 7.5, and 3.6 µM against HT-29 cells. In mitochondrial transmembrane potential assay, compounds 3, 7, and 8 showed ED50s of 8.5, 75 and 310 nM, respectively. (10)
Availability
Wild-crafted.
|

![]()



 Gen info
Gen info
 Uses
Uses